 ATM 107 The
Oceans
ATM 107 The
Oceans
Wed., 7
October 2015
Daily Announcements:
1)
Exam #1
grades are posted on Blackboard.
Song #1: “Bridge
Over Troubled Water” by Simon & Garfunkel
Song #2: “Have
You Ever Seen the Rain” by Creedence Clearwater Revival
Text Source Today: 6.2, 6.3, 6.4
·
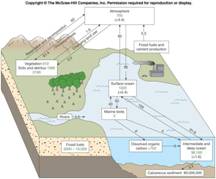
Oxygen (O2),
Carbon Dioxide (CO2) and pH Notes Template
·
Carbon Dioxide Cycle PDF DOC
·
Oxygen Balance
·
Other
Constituents
Study Guide Material (Stacey’s Guide) :
1)
How do biological
activities in the oceans affect the vertical distribution of oxygen
and carbon dioxide in the
oceans? What is meant by “compensation
depth?”
2)
How does CO2
act as a “buffer” in the oceans?
3)
What
is meant by “pH?” How does seawater’s pH rate against other substances?
4)
Is seawater
generally “acidic” or “alkaline?”
5)
Describe the
“carbon dioxide cycle.”
6)
Explain the
“oxygen balance” in the oceans.
7)
What “other
substances” are in the oceans? What are
nutrients?
8)
Where is the most
saline body of water on Earth, outside
9)
Where is the most
saline lake on Earth (including
10)
What is so unusual about